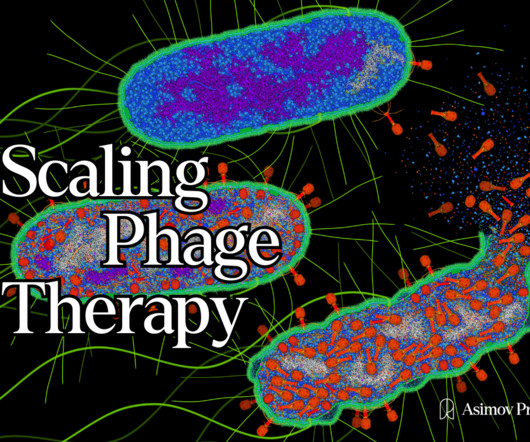
Scaling Phage Therapy
Codon
FEBRUARY 13, 2024
Tom Ireland writes about the companies and technologies that are reimagining phage therapy. Soon after its publication, scientists, journalists, and investors were revisiting ‘phage therapy’ as a promising alternative to our failing antibiotics. Read it on our website here. Illustration by David S. Always free.















































Let's personalize your content