NOW AVAILABLE: The 2022 Economic Report on U.S. Pharmacies and Pharmacy Benefit Managers
Drug Channels
MARCH 15, 2022
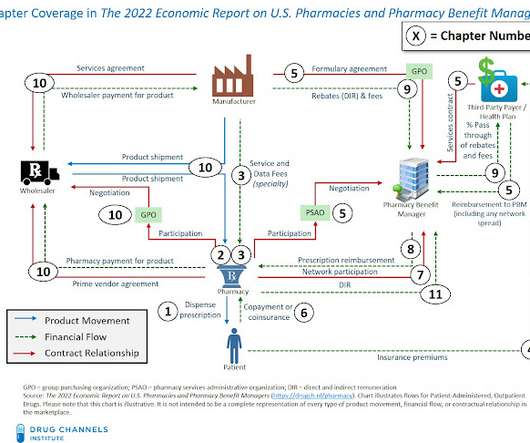
Pharmacies and Pharmacy Benefit Managers , available for purchase and immediate download. Pharmacies and Pharmacy Benefit Managers , our 13th edition, provides a comprehensive, fact-based, and non-partisan tool for understanding the entire U.S. I am pleased to announce our new 2022 Economic Report on U.S.















































Let's personalize your content