CureVac cuts jobs, licenses out vaccines to GSK
BioPharma Drive: Drug Pricing
JULY 3, 2024
The mRNA specialist plans to eliminate 30% of its workforce as part of a restructuring that will prioritize “high-value” projects like its cancer vaccines.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
JULY 3, 2024
The mRNA specialist plans to eliminate 30% of its workforce as part of a restructuring that will prioritize “high-value” projects like its cancer vaccines.
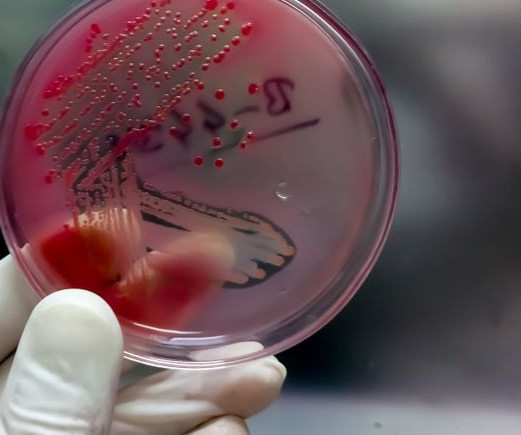
BioPharma Drive: Drug Pricing
JULY 20, 2023
The startup will take on development of a shigellosis vaccine GSK inherited when it acquired LimmaTech’s predecessor, GlycoVaxyn, in 2015.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Discovery Today
JULY 7, 2022
Pfizer and Touchlight agree to patent license for Pfizer to utilise rapid, scalable, enzymatic doggybone DNA (dbDNA) in Pfizer’s clinical and commercial manufacture of its mRNA vaccines, therapeutics, and gene therapiesAgreement includes upfront payment, potential development and commercial milestone payments, and royalties upon commercializationAccess (..)

The Pharma Data
JULY 18, 2021
Food and Drug Administration (FDA) granted Priority Review designation for the Biologics License Application (BLA) for their mRNA vaccine to prevent COVID-19 in individuals 16 years of age and older. The Pfizer-BioNTech COVID-19 vaccine, which is based on BioNTech proprietary mRNA technology, was developed by both BioNTech and Pfizer.

The Pharma Data
MAY 7, 2021
The Pfizer-BioNTech COVID-19 Vaccine is currently available in the U.S. Since then, the companies have delivered more than 170 million doses of the vaccine across the U.S. We are proud of the tremendous progress we’ve made since December in delivering vaccines to millions of Americans, in collaboration with the U.S.
The Pharma Data
AUGUST 13, 2020
The UK government has signed deals for a further 90 million doses of coronavirus vaccine. The vaccines are being developed by the Belgian pharmaceutical company Janssen and the US biotech company Novavax. It means the UK has placed orders for six experimental vaccines, taking its potential stockpile to 340 million doses.

The Pharma Data
NOVEMBER 3, 2020
The Coalition for Epidemic Preparedness Innovations (CEPI) has agreed to fund development of a potential COVID-19 vaccine from China’s Clover Biopharmaceuticals with a global phase 2/3 study and through licensing and distribution. million investment announced in July. Earlier this year, CEPI provided $3.5
The Pharma Data
MARCH 14, 2021
Following a recent concern raised around thrombotic events, AstraZeneca would like to offer its reassurance on the safety of its COVID-19 vaccine based on clear scientific evidence. Safety is of paramount importance and the Company is continually monitoring the safety of its vaccine. COVID-19 Vaccine AstraZeneca , formerly AZD1222.

The Pharma Data
JANUARY 5, 2021
Due to the unforeseen delays, Targovax has extended the term of IOvaxis’s license option by 3 months, otherwise the agreement remains unchanged. To accommodate the delay caused by these unforeseen circumstances, Targovax has granted to IOVaxis an extension to the license option period by 3 months.

The Pharma Data
SEPTEMBER 30, 2021
Anderson Professor of Chemical and Biomolecular Engineering, and his colleagues, are reporting in iScience the event of an intranasal subunit vaccine that gives durable local immunity against inhaled pathogens. A fundamental limitation of intramuscular vaccines is that they’re not designed to elicit mucosal immunity.

The Pharma Data
JUNE 24, 2021
The primary objective in the trial is to describe safety when both vaccines are co-administered, with follow up six months after vaccination. Secondary objectives are to describe immune responses produced by each of the vaccines. Secondary objectives are to describe immune responses produced by each of the vaccines.

The Pharma Data
DECEMBER 19, 2020
Nasdaq: MRNA) a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines today announced that the U.S. 11 ACIP members voted in favor of the vaccine and 0 members voted against. We look forward to vaccinations of this important population starting this week.”. .–( BUSINESS WIRE )– Moderna, Inc.,

The Pharma Data
NOVEMBER 13, 2020
WHO today listed the nOPV2 vaccine (Bio Farma, Indonesia) for emergency use to address the rising cases of a vaccine-derived polio strain in a number of African and East Mediterranean countries. The emergency use listing, or EUL, is the first of its kind for a vaccine and paves the way for potential listing of COVID-19 vaccines.

The Pharma Data
AUGUST 10, 2020
Novavax is partnering up with the Serum Institute of India Private Limited (SIIPL) in a licensing deal to development and marketing of up to one billion doses of its potential recombinant COVID-19 vaccine candidate, NVX?CoV2373. Negotiations are currently ongoing to have SIIPL manufacture the candidate within India. M adjuvant.
The Pharma Data
AUGUST 28, 2020
The government plans to train up an army of health professionals to be ready to give the coronavirus vaccine, if and when one is shown to work. This could include pharmacists, who already deliver flu vaccines, midwives and physiotherapists. But a vaccine is not expected to be ready before Christmas. Source link.

The Pharma Data
OCTOBER 6, 2021
First study to investigate the safety and immunogenicity of both vaccines when co-administered compared to each vaccine administered separately in adults aged 65 years and older Timely new data for the start of the influenza vaccination campaigns across the Northern Hemisphere. About the study.

The Pharma Data
AUGUST 6, 2020
We pointed out last week that China was a notable missing element in AstraZeneca’s COVID-19 vaccine strategy, despite being the drugmaker’s second-largest market. The pair will also explore the possibility of producing the vaccine for other markets. The vaccine was moved into phase 2/3 in May. That has changed.
The Pharma Data
SEPTEMBER 4, 2020
Dr Moncef Slaoui, the leading doctor involved with Operation Warp Speed, has said it is unlikely a vaccine for the US will be ready by November. . But Slaoui did say that he believes a vaccine will be available by the end of the year, and could possibly vaccinate between 20 and 25 million people.

The Pharma Data
DECEMBER 23, 2020
company developing UB-612 a multitope peptide-based vaccine to fight COVID-19, today announced an exclusive agreement with Aurobindo Pharma to expand its global development and commercialization of UB-612 to India and the United Nations Children’s Fund (UNICEF) agency. billion, to deliver the vaccine in multiple countries.

The Pharma Data
JANUARY 22, 2021
As research developments into RNA vaccines help scientists accelerate drug candidates to arm the immune system against coronavirus, Pharma IQ ’s Keeping tabs on Covid-19 update returns with news from some of the biotechnology innovators leading the fight against the global pandemic.
The Pharma Data
MARCH 2, 2021
Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) has recommended the first single-shot COVID-19 vaccine, developed by the Janssen Pharmaceutical Companies of Johnson & Johnson, for individuals 18 years of age and older under the Emergency Use Authorization (EUA) issued by the U.S. “For
The Pharma Data
MARCH 11, 2021
Sanofi Pasteur, the vaccines global business unit of Sanofi, and Translate Bio (NASDAQ: TBIO), a clinical-stage messenger RNA (mRNA) therapeutics company, today announced the start of the Phase 1/2 clinical trial for MRT5500, an mRNA vaccine candidate against SARS-CoV-2, the virus that causes COVID-19.
The Pharma Data
APRIL 1, 2021
vaccine efficacy observed against COVID-19, measured seven days through up to six months after the second dose. Vaccine was 100% effective in preventing severe disease as defined by the U.S. Vaccine was 100% effective in preventing COVID-19 cases in South Africa, where the B.1.351 Food and Drug Administration.

The Pharma Data
MAY 4, 2021
. Pfizer plans to file for full FDA approval of Covid vaccine at the end of this month ( CNBC ). The FDA is set to authorize the Pfizer-BioNTech vaccine for those 12-15 years old by early next week. Pfizer Reaps Hundreds of Millions in Profits From Covid Vaccine ( NYTimes ) ( WSJ ) ( Endpoints ) ( Reuters ).

The Pharma Data
DECEMBER 11, 2020
FDA authorizes COVID-19 mRNA vaccine for emergency use; companies are prepared to deliver first doses in the U.S. In collaboration with Operation Warp Speed, Pfizer and BioNTech, as well as other vaccine companies are expected to deliver hundreds of millions of vaccine doses to Americans by the end of 2021.

The Pharma Data
MARCH 26, 2021
The new data is a testament to the companies’ ongoing commitment to developing this vaccine further and collecting data in order to support broader and more flexible vaccine distribution and inoculation. From the beginning our goal was to make our vaccine broadly available to people around the world. Source link:[link].

The Pharma Data
MAY 6, 2021
Under the MoU, the companies and the IOC will coordinate with National Olympic Committees (NOCs) around the world to understand and work to help address the local need for vaccine doses for national delegations’ participation in the Games. We are proud to play a role in providing vaccines to athletes and national Olympic delegations.”.

The Pharma Data
JANUARY 31, 2021
Gilead and Gritstone will develop an HIV-specific therapeutic vaccine using Gritstone’s proprietary prime-boost vaccine platform, comprised of self-amplifying mRNA (SAM) and adenoviral vectors, with antigens developed by Gilead. This press release features multimedia. View the full release here: [link].
The Pharma Data
JUNE 26, 2021
Sanofi and Translate Bio initiate Phase 1 clinical trial of mRNA influenza vaccine. The trial will evaluate the safety and immunogenicity of a monovalent flu vaccine candidate coding for the hemagglutinin protein of the A/H3N2 strain of the influenza virus. JUNE 22 , 2021. We look forward to sharing initial results by year-end. ”. “

The Pharma Data
JANUARY 6, 2021
(Nasdaq: MRNA), a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, today announced that the European Commission has granted a conditional marketing authorization (CMA) for COVID-19 Vaccine Moderna, allowing vaccination programs using the Moderna vaccine to be rolled out across the European Union.

The Pharma Data
NOVEMBER 17, 2021
AstraZeneca and its partners have released for supply two billion doses of their COVID-19 vaccine to more than 170 countries across every continent on the planet in the last 11 months. 2) From the body of evidence in clinical trials and real-world data, the vaccine has been shown to have an acceptable safety profile.(3,4,5,6,7).
The Pharma Data
SEPTEMBER 28, 2021
Many parents have questions on COVID-19 and when vaccines are going to be available for youngsters younger than 12 years aged. We are therefore also wanting to see COVID-19 vaccines available for young children. Some have stated that they’re still enrolling, and a few are still administering doses or following participants.
The Pharma Data
JULY 23, 2021
government has purchased an additional 200 million doses of the Pfizer-BioNTech COVID-19 Vaccine. government with 500 million doses of the companies’ COVID-19 vaccine for donation to the world’s poorest nations. government in the fight against this pandemic, we are proud of the impact of vaccination efforts across the country.

The Pharma Data
MAY 4, 2021
The New Zealand Medicines and Medical Devices Safety Authority (Medsafe) has banned the trade in unapproved COVID-19 vaccines for a year to prevent the use of vaccines that may not be safe or effective. . The public could also import personal supplies of vaccines. Medsafe framed the action in the context of public safety. . “In

The Pharma Data
JANUARY 12, 2021
Nasdaq: MRNA), a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, today announced that Swissmedic, the Swiss Agency for Therapeutic Products, has authorized the COVID-19 Moderna Vaccine in Switzerland. million doses of the COVID-19 Vaccine Moderna. About the COVID-19 Vaccine Moderna.
The Pharma Data
AUGUST 3, 2021
Accelerates development of current Sanofi licensed programs in vaccines and potential to explore other therapeutic areas Fast tracks establishment of Sanofi’s recently announced mRNA Center of Excellence Full integration upgrades drug formulation capabilities and enhances US talent in a promising new technology.
The Pharma Data
AUGUST 16, 2021
Food and Drug Administration (FDA) to support the evaluation of a third, or booster, dose of the companies’ COVID-19 vaccine (BNT162b2) for future licensure. Vaccination is our most effective means of preventing COVID-19 infection – especially severe disease and hospitalization – and its profound impact on protecting lives is indisputable.

The Pharma Data
JUNE 24, 2021
This follows the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) positive opinion to authorize the vaccine in this age group. COMIRNATY was the first COVID-19 vaccine to receive authorization in the EU and is the first to have its CMA extended to adolescents. g doses of the COVID-19 vaccine.

The Pharma Data
JULY 21, 2021
(NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced the signing of a letter of intent with The Biovac Institute (Pty) Ltd, known as “Biovac,” a Cape Town-based, South African biopharmaceutical company, to manufacture the Pfizer-BioNTech COVID-19 Vaccine for distribution within the African Union. This press release features multimedia.

The Pharma Data
SEPTEMBER 12, 2020
Food and Drug Administration to expand the enrollment of their Phase 3 pivotal COVID-19 vaccine trial to up to approximately 44,000 participants which also allows for the enrollment of new populations. (NYSE: PFE) and BioNTech SE (NASDAQ: BNTX) announced today that they have submitted an amended protocol to the U.S.

The Pharma Data
DECEMBER 23, 2020
24, 2020 /PRNewswire/ — COVAXX’s UB-612 is the first multitope, synthetic peptide-based COVID-19 vaccine candidate in clinical trials and it utilizes normal refrigeration (no freezing required) for distribution. COVAXX is currently conducting a Phase 1 clinical trial for the vaccine candidate. .

The Pharma Data
OCTOBER 19, 2020
.–( BUSINESS WIRE )– Merck (NYSE: MRK), known as MSD outside the United States and Canada, today announced findings from two additional Phase 3 studies evaluating the safety, tolerability and immunogenicity of V114, the company’s investigational 15-valent pneumococcal conjugate vaccine. among adults 65 years of age or older.

The Pharma Data
NOVEMBER 22, 2021
Pfizer-BioNTech vaccine demonstrated 100 efficacity against COVID-19 in longer- term analysis, with no serious safety enterprises linked. Data will support planned cessions for full nonsupervisory blessing of the vaccine in this age group in theU.S. and worldwide NEW YORK & MAINZ, Germany– (BUSINESS WIRE)– PfizerInc.

The Pharma Data
FEBRUARY 24, 2022
If the EC grants the variation, the decision will be immediately applicable to all 27 EU member states, making booster vaccines available to everyone 12 years and older. . The COVID-19 vaccine booster for individuals 12 through 15 years of age was already granted Emergency Use Authorization by the U.S. and Europe.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content