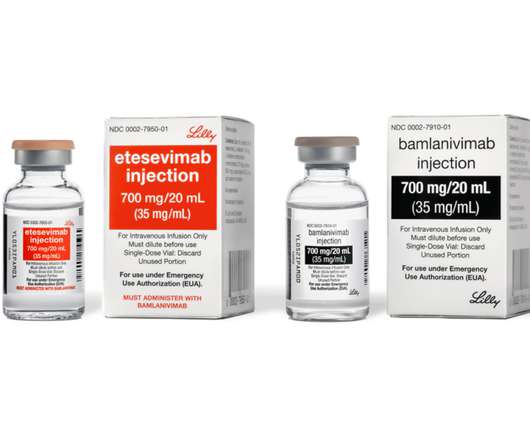
Bamlanivimab alone with the U.S. government and is focusing on supply of bamlanivimab and etesevimab together
The Pharma Data
APRIL 14, 2021
government for its neutralizing antibody therapies authorized for emergency use as a treatment for COVID-19. Lilly subsequently developed bamlanivimab and etesevimab for administration together, in order to meet the potential challenge of SARS-CoV-2 variants likely to resist treatment with either monoclonal antibody used alone.












Let's personalize your content