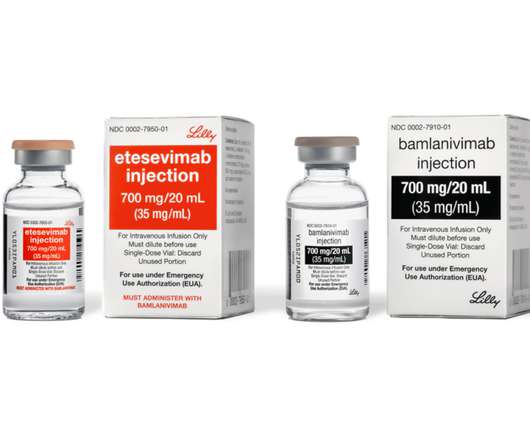
Bamlanivimab alone with the U.S. government and is focusing on supply of bamlanivimab and etesevimab together
The Pharma Data
APRIL 14, 2021
Adverse events reported in at least 1% of BLAZE-1 clinical trial participants on bamlanivimab 700 mg alone or placebo were nausea (3% vs 4%), diarrhea (1% vs 5%), dizziness (3% vs 2%), headache (3% vs 2%), pruritus (2% vs 1%) and vomiting (1% vs 3%). volunteers to evaluate the safety, tolerability, pharmacokinetics and immunogenicity.












Let's personalize your content