Pfizer to pull sickle cell drug from market, shut down trials
BioPharma Drive: Drug Pricing
SEPTEMBER 26, 2024
The withdrawal comes as trial results indicate safety concerns with the drug, Oxbryta, which was first approved in the U.S.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

BioPharma Drive: Drug Pricing
SEPTEMBER 26, 2024
The withdrawal comes as trial results indicate safety concerns with the drug, Oxbryta, which was first approved in the U.S.

Drugs.com
MARCH 11, 2024
MONDAY, March 11, 2024 -- Following disappointing trial results, the maker of a controversial ALS drug may pull the medication off the market. In a statement issued Friday, Amylyx Pharmaceuticals said that Relyvrio failed to help patients in a.

ProRelix Research
DECEMBER 27, 2022
Clinical trials form the crux of all regulatory decisions regarding any new health intervention such as drugs and medical devices and thus require to be performed with the utmost care […]. The post USA Clinical Trial Market Size and Opportunities appeared first on ProRelix Research.

Antidote
MAY 10, 2024
While it is most commonly used in relation to art generators or web searches, AI has many practical applications beyond these uses — especially within the clinical trial marketing space.

ProRelix Research
NOVEMBER 2, 2023
Although clinical trials provide useful information regarding the efficacy and safety of new drugs, biological products, and medical devices, the information captured through them is not all-encompassing. Limited patient populations, […] The post Post-Marketing Surveillance Strategies appeared first on ProRelix Research.

Drugs.com
APRIL 4, 2024
THURSDAY, April 4, 2024 (HealthDayNews) -- Following disappointing trial results, the maker of a controversial ALS drug said it is pulling the medication off the market. In a statement issued Thursday, Amylyx Pharmaceuticals said that Relyvrio.

Antidote
APRIL 4, 2024
Before it is released onto the market, the development of any new drug or medical device must undergo rigorous testing , part of which involves clinical trials. Clinical trials are integral to making sure that any new therapy is both safe and effective for individuals, and volunteers are a vital part of the process.

Antidote
SEPTEMBER 25, 2023
Clinical trials are how researchers advance their knowledge about potential new treatments, including medications, medical devices, and lifestyle interventions. Clinical trials are divided into phases , each with a distinct duration, purpose, and number of volunteers needed.

Antidote
OCTOBER 5, 2023
However, connecting with the right patient population is one of the biggest challenges facing clinical trials today so employing strategic clinical trial recruitment advertising strategies is imperative. In order to conduct successful medical research, it is essential to identify and enroll the right participants.

Antidote
AUGUST 10, 2023
In the age of the internet, effective clinical trial digital advertising is imperative to finding the right participants for a research study. Being able to reach patients where they are using outreach material that resonates is vital — but it doesn’t happen without smart, data-driven strategies.

BioPharma Drive: Drug Pricing
NOVEMBER 5, 2024
Rallybio is headed into a mid-stage trial with enough cash to last it through 2026, but biotech's down market has made the company's journey difficult.

ProRelix Research
NOVEMBER 5, 2024
Clinical trials are necessary in advancing medical research and health care options by allowing new treatments to be introduced in the market through safety and efficacy testing in humans. Successful […] The post Clinical Trials and Human Subject Protection as per US FDA appeared first on ProRelix Research.

ProRelix Research
JULY 6, 2023
Biosimilar clinical trials and study designs’ Considerations The rising prevalence and large market share of biological products emphasize their importance as treatment options for several cancers and autoimmune diseases.

Drugs.com
MARCH 11, 2024
MONDAY, March 11, 2024 -- Following disappointing trial results, the maker of a controversial ALS drug may pull the medication off the market.In a statement issued Friday, Amylyx Pharmaceuticals said that Relyvrio failed to help patients in a.

The Premier Consulting Blog
OCTOBER 21, 2024
However, accelerated approval comes with a condition: sponsors must conduct confirmatory trials to verify the drug’s anticipated clinical benefits using robust outcome measures. Concerns have arisen over delays—sometimes spanning over 7–8 years—that may expose patients to risks before confirmatory trials are completed.

FDA Law Blog: Biosimilars
SEPTEMBER 26, 2024
In fact, it has a whole page dedicated to “CDRH Innovation,” which states that CDRH “is committed to advancing public health by helping to bring innovative technologies to market.” Manufacturers can expect increased interaction with the review team and prioritized review of the marketing submission.”

PPD
AUGUST 28, 2024
For pharmaceutical innovators and drug developers working to bring oncology therapies to market, patients are the “why” behind it all. For many patients, involvement in oncology clinical trials represents a last hope for an effective therapy. Many of these patients’ conditions are disabling.

Reprocell
NOVEMBER 14, 2024
To address the issues of time and cost, pharmaceutical companies rely on central laboratories (CLs) for consistency, organization, and efficiency during the clinical trial phase, which is essential for drug approval.

PPD
AUGUST 16, 2023
The COVID-19 pandemic rapidly accelerated the adoption of hybrid and decentralized clinical trial (DCT) models. However, as the world settles into its post-pandemic state and returns to pre-pandemic paradigms in many areas, the pharmaceutical industry remains dedicated to moving beyond traditional, centralized clinical trial constructs.

Antidote
JANUARY 3, 2023
Before becoming available to patients, every medication, therapy, and intervention must undergo a rigorous approval process: the clinical trial. Clinical trials are divided into phases that are aimed at testing the safety and efficacy of every potential new treatment before it is able to enter the market.

Advarra
OCTOBER 1, 2024
Managing clinical trial budgets efficiently is necessary for the success and sustainability of clinical research sites. Effective budget management not only ensures trials are financially viable but also maximizes return on investment (ROI). the impact and value of the data produced).

Antidote
OCTOBER 25, 2024
Antidote’s Senior Manager of Digital Marketing, Sean Irvine, shared his thoughts on the importance of health literacy and how we can create more patient-first recruitment campaigns. October is National Health Literacy Month, a time to focus on how we can improve a person’s understanding of the health and medical information shared with them.

Antidote
SEPTEMBER 13, 2023
Globally, there are thousands of research studies and clinical trials conducted each year, all with the shared goal of bringing new treatment options and medical devices to the market. However, approximately 80% of clinical trials are delayed or closed because of problems with recruitment.

PPD
OCTOBER 8, 2024
With FSO, all clinical trial tasks (e.g., In a hybrid model, one or more FSP offerings are added to a new or existing FSO arrangement to optimize clinical trial operations or address unforeseen circumstances and changing demands. study startup, data management, clinical monitoring, pharmacovigilance, regulatory affairs, etc.)
Conversations in Drug Development Trends
APRIL 3, 2024
Are you aware of the challenges you must address for a successful radiopharmaceutical trial? Enhancing Patient Participation in Radiopharmaceutical Trials Patient recruitment is a critical yet challenging part of radiopharmaceutical trials.

The Premier Consulting Blog
NOVEMBER 12, 2024
Recently, we convened a panel of national payer pharmacy decision-makers to ask them about how they evaluate 505(b)(2)s and whether an approved FDA label is enough to secure strong payer market access. This blog is the first installment in a series summarizing our findings. Non-preferred coverage.

Antidote
JULY 9, 2024
Though clinical trials are often discussed in the context of medications and therapies, the devices that administer these medications must also be approved by the FDA before they can be released to market.

Fierce BioTech
MAY 17, 2024
Genetic Counseling Accelerates Trial Success pesurya Fri, 05/17/2024 - 19:26 Wed, 06/26/2024 - 14:00 Resource Type Webinar Ashley Cannon, PhD, MS, CGC Duration 60 Minutes Telehealth genetic counseling is a proven method of clinical genomics care delivery, but little has been published regarding its use to support clinical trials.

PPD
JULY 6, 2023
It’s estimated that nearly three out of every four clinical trials are conducted by contract research organizations (CROs), highlighting just how much sponsors value — and rely on — the work that CROs perform. Surveys show that CROs improve trial efficiency and increase productivity.

Conversations in Drug Development Trends
DECEMBER 5, 2023
Author: Lona Sheeran, SVP, Clinical Operations, Early Phase At this year’s Clinical Trials Nexus, I had the privilege of representing Worldwide Clinical Trials as the sole CRO on a panel discussion: “Reversing the Conversation: What the Clinical Trial Industry Really Wants from its Service Providers.”

Agency IQ
JUNE 28, 2024
BY COREY JASEPH, MS, RAC New guidance from the European Commission outlines alternatives for full pre-market clinical trials for orphan devices, defined by the Commission for the first time. Notably, this is also the first guidance to describe the possible use of a conditional Notified Body certificate.

Vial
FEBRUARY 29, 2024
Contract research organizations (CROs) are an integral partner of the drug development process, as they play a pivotal role supporting clinical trial conduct for pharmaceutical, biotechnology, and medical device sponsor companies. That is, how many clinical trials are actually managed by these organizations? between 2023 and 2032.

Predictive Oncology
SEPTEMBER 1, 2023
“Pamela’s unwavering passion for a cancer cure paved the way for an incredible opportunity to directly make a difference in the lives of countless people,” Molecule to Market podcast. In this episode of Molecule to Market, host Raman Sehgal talks with Pamela Bush, Ph.D.,

PPD
AUGUST 14, 2024
Clinical trials have significantly increased in complexity over the last 20 years, creating new challenges. Patients are eager to participate in trials but remain largely unaware of their availability despite growing efforts to recruit. The increase in complexity isn’t just creating challenges for patients.

The Pharma Data
NOVEMBER 26, 2020
Analyzing the benefits and challenges of implementing direct-to-patient services in the clinical supply chain to help meet patient requirements, improve supply chain efficiency and accelerate speed to market. Add bookmark. Download Your Copy. The benefits of developing a patient-centric clinical supply chain.

Vial
JUNE 18, 2024
The success of clinical trials hinges on increasing access to participation by all eligible patients, including populations that have been underrepresented due to the barriers highlighted below. Representation, Underrepresentation & Diversity in Clinical Trials Studies indicate a need to increase patient accessibility to clinical trials.

Antidote
FEBRUARY 22, 2024
Behind every new treatment is the incredible team of doctors, researchers, patients, and clinical trial specialists who worked on the research that made it possible.
Antidote
SEPTEMBER 1, 2023
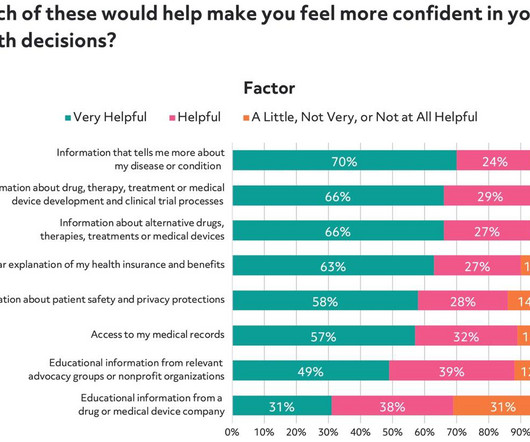
As part of our efforts to better understand patients and their motivations to participate in clinical trials, Antidote and SCORR Marketing partnered on a survey to gain a deeper understanding of how patients think about the medical research process.

Antidote
JUNE 22, 2023
Creating clinical trial marketing materials is a delicate balance — it's important to craft outreach that connects with patients and care partners, but it’s also important to adhere to the guidelines set out by the Food and Drug Administration (FDA) to receive the approval of the Internal Review Board (IRB).

BioPharma Drive: Drug Pricing
APRIL 4, 2024
In the wake of a major trial failure, the company is withdrawing Relyvrio and cutting costs with a restructuring that involves laying off 70% of its employees.

Antidote
APRIL 9, 2024
Understanding how to recruit patients for clinical trials can be one of the most challenging aspects of performing medical research. Additionally, using a mix of outreach is typically the best strategy to reach the highest number of potentially qualified patients — meaning observing best practices across marketing disciplines is advised.

Conversations in Drug Development Trends
AUGUST 23, 2023
Patients are the backbone of clinical trials, playing an essential role in the drug development process. This engagement is often less understood and is underutilized by sponsors, meaning a significant element of the trial and drug experience is missed during sponsor engagement with the FDA.

Antidote
FEBRUARY 12, 2024
A common problem in the clinical trial industry is finding enough interested individuals to take part in a study. However, keeping patients at the center of clinical trial marketing is one of the most effective ways to address this issue.

Vial
MAY 13, 2024
However, progress from molecule to approved drug is hampered by extremely high costs and lengthy clinical trials , and approximately 90% of drugs that reach clinical trials fail. only 5% of molecules in oncology Phase I trials reach the market taking, on average, 7.5 According to Rodríguez-Molinero et al.,
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content