The future of drug discovery: Using AI to optimise the hit-to-lead process
Drug Discovery World
AUGUST 20, 2024
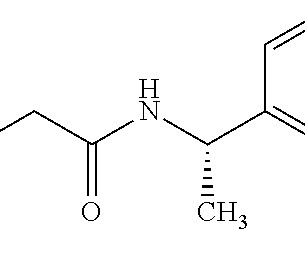
Mirit Eldor , Managing Director, Life Sciences Solutions, Elsevier, looks at how artificial intelligence (AI) can improve the hit-to-lead process for small molecules. It’s therefore no surprise that pharmaceutical companies are increasingly interested in using artificial intelligence (AI) to accelerate development and reduce costs.














Let's personalize your content