The Data-Driven Future of Drug Development
DrugBank
OCTOBER 17, 2024
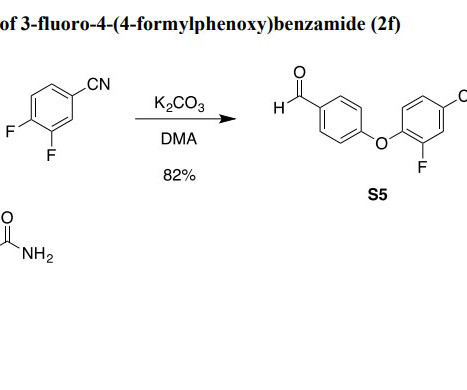
By harnessing the vast amounts of data generated throughout the development pipeline, pharmaceutical companies can accelerate the discovery of novel therapies, optimize clinical trial design, enhance drug safety monitoring, and deliver personalized medicine, ultimately improving patient outcomes and transforming the future of healthcare.

























Let's personalize your content