Antisense therapy restores fragile X protein production in human cells
Science Daily: Pharmacology News
JULY 5, 2023
An antisense therapy restores production of the protein FMRP in cell samples taken from patients with fragile X syndrome.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Science Daily: Pharmacology News
JULY 5, 2023
An antisense therapy restores production of the protein FMRP in cell samples taken from patients with fragile X syndrome.

Drugs.com
JUNE 25, 2024
TUESDAY, June 25, 2024 -- An experimental stem cell therapy can essentially cure type 1 diabetes by restoring insulin production in some patients, early clinical trial results show.Seven out of 12 patients no longer needed daily insulin shots after.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

BioPharma Drive: Drug Pricing
DECEMBER 2, 2024
Next-generation sequencing allows for critical insights into gene therapy products, which can help streamline and accelerate everything from process development and production to regulatory approval.

BioPharma Drive: Drug Pricing
DECEMBER 6, 2023
The funds are meant to boost Fujifilm’s capacity to manufacture cell therapies, a market it expects to grow substantially in the coming years.
BioPharma Drive: Drug Pricing
AUGUST 24, 2023
Bristol Myers Squibb is among those backing the startup, which claims the manufacturing capacity at its New Jersey plant can surpass that of conventional CDMO facilities.

Drug Target Review
FEBRUARY 10, 2025
In the rapidly advancing field of cell therapies, Dr Jason Bock has emerged as a leader, known for his innovative approach to optimising the development process. With over 25 years of experience in therapeutics, Bock has played a pivotal role in shaping the future of cell therapies, particularly through his work at CTMC.

BioPharma Drive: Drug Pricing
OCTOBER 26, 2023
Executives at the biotech say they’re trying to get ahead of the payer and production challenges that will face their gene editing treatment exa-cel, which is now under FDA review.

FDA Law Blog: Drug Discovery
NOVEMBER 10, 2024
Food and Drug Administration (FDA) plays a pivotal role in fostering the development of treatments for rare diseases through its Orphan Products Grants Program. Each year, FDA selects a limited number of clinical trials to fund to help sponsors pursue development of medical products for rare diseases and advance their field.
Conversations in Drug Development Trends
NOVEMBER 12, 2024
By Amy Raymond, PhD, PMP, Executive Director, Therapeutic Strategy Lead, Rare Disease Cell and gene therapies (CGTs) include cutting-edge approaches that offer the hope of a healthier, happier, and better tomorrow for a wide range of patient populations. Below, we discuss some of these challenges in cell therapy trials.

Science Daily: Pharmacology News
OCTOBER 16, 2024
Scientists have discovered a new process in our immune systems that leads to the production of an important family of anti-viral proteins called interferons.

BioPharma Drive: Drug Pricing
SEPTEMBER 4, 2024
A planned facility in California will boost Novartis’ West Coast supply chain as new uses for the company’s radioligand therapies grow.

The Premier Consulting Blog
NOVEMBER 6, 2024
Inhaled combination products (ICP) have emerged as a significant advancement in the treatment of respiratory diseases such as asthma, chronic obstructive pulmonary disease (COPD), and other pulmonary conditions. However, these products present unique challenges from a Chemistry, Manufacturing, and Controls (CMC) standpoint.
Broad Institute
MAY 16, 2024
Gene therapy could potentially treat a range of severe genetic brain disorders, which currently have no cures and few treatment options. Since we came to the Broad we’ve been focused on the mission of enabling gene therapies for the central nervous system,” said Deverman, senior author on the study. “If
BioPharma Drive: Drug Pricing
AUGUST 11, 2023
Filed in Maryland district court, the lawsuit claims the biotech unjustly profited from using HeLa cells to develop AAV vectors for its gene therapy products.
Drug Target Review
NOVEMBER 8, 2023
Problem w/ CTs and foundational understanding of Vittoria: can you explore the current limitations of cell therapies and the challenges faced by patients and providers? Currently, only a small percentage of cancers can be effectively treated with cell therapies, and there is little diversity in the currently approved products.

PPD
OCTOBER 17, 2024
Cell and gene therapy (CGT) studies are rapidly gaining momentum in the Asia-Pacific region, fueled by growing patient demand and a thriving ecosystem of innovation. In China, the high incidence of solid tumors is driving an urgent need for advanced therapies, spurring the push for new treatment approaches.
Drug Target Review
JANUARY 4, 2024
What distinguishes Alder Therapeutics’ approach to regenerative cell therapy development from traditional methods, and how does it aim to reduce risks in the preclinical phase? Traditional approaches to regenerative cell therapy development are defined by several challenges. For manufacturing, it’s no different.

LifeSciVC
APRIL 24, 2024
Additionally, for illustrative reasons this is geared towards a single target / product focus vs. broader platform diligence, though many of these mental models will apply for selecting targets and indications for a platform. with gene editing or gene therapy, enzyme replacement therapy), agonism (e.g., in liver, in CNS)?

SugarCone Biotech
MARCH 2, 2025
Less clear is whether we can productively and safely inhibit TGF- activity at all given the toxicity issues associated with TGF- inhibition. Where TGF- is present there is no or limited IFN- secretion by T cells and that means no PD-L1 expression within the tumor microenvironment, aka the TME.

FDA Law Blog: Drug Discovery
DECEMBER 7, 2022
Valentine — On November 22, 2022, FDA approved CSL Behring’s BLA for Hemgenix (etranacogene dezaparvovec), an AAV-based gene therapy for the treatment of adults with Hemophilia B who currently use Factor IX prophylaxis therapy, have current or historical life-threatening hemorrhage, or have repeated, serious spontaneous bleeding episodes.
Drug Target Review
FEBRUARY 9, 2024
What key findings about stem cell behaviour, differentiation and integration within host tissues impact the development of stem cell therapies? Despite their potential, ADSC therapy faces several challenges in preclinical studies. Is there a certain disease or condition that you believe stem cell therapy holds the most promise for?

Drug Discovery Today
DECEMBER 17, 2019
.– December 10, 2019 — Catalent, the leading global provider of advanced delivery technologies, development, and manufacturing solutions for drugs, biologics, gene therapies, and consumer health products, today announced that it has completed clinical production of Bridge Therapeutics Inc.’s
Drug Target Review
AUGUST 31, 2023
Stem cell therapies have already demonstrated their prowess in treating diverse cancers and ailments linked to the blood and immune system. Chronic inflammation involving microglia has been linked to the disease, as the release of inflammatory molecules triggers an increase in β-amyloid production.

DrugBank
JUNE 13, 2024
Gene Therapy: Reprogramming the Body's Cellular Code Gene therapy is an exciting field that treats diseases at their genetic roots. A key part of gene therapy is efficiently delivering the therapeutic genetic material directly into target cells.

Drug Discovery Today
DECEMBER 5, 2020
OVO Biomanufacturing, a spin-out from the University of Warwick and Coventry University, is developing digital solutions to improve the efficiency of viral vaccine and gene therapy manufacture. The technology can be applied to any virus that is grown to produce a vaccine or therapy.

The Premier Consulting Blog
MARCH 7, 2024
The FDA’s January 2020 guidance, Chemistry, Manufacturing and Control (CMC) [1] Information for Human Gene Therapy Investigational New Drug Applications (INDs), outlines the analytical methods that define the quality, safety and efficacy of gene therapy therapeutics.

Drug Discovery Today
NOVEMBER 5, 2020
London, November 4th, 2020: NanoMab Technology Limited, a privately held biopharmaceutical company focussing on cancer precision therapies, today announced that it had received CTA Acceptance from the Medicines Healthcare products Regulatory Agency (MHRA) to carry out a Phase II clinical Study for its NM-01 product.

Drug Patent Watch
DECEMBER 9, 2024
High-Throughput Screening: Modern Technology Meets Natural Products Advanced technologies now allow researchers to rapidly test thousands of natural compounds against specific disease targets. The development of Taxol involved multiple patents, including those for the isolation method, synthetic production, and various formulations.
BioPharma Drive: Drug Pricing
JULY 26, 2023
The startup is the latest young company to emerge with plans to make production of cell-based medicines easier for researchers and biotechs.

Drug Channels
OCTOBER 24, 2024
Shabbir explains the barriers that providers face when dealing with branded portals for multiple products. He then maintains that patients can access new therapies more quickly when the manufacturer relies on a brand-agnostic hub connected to a large network of providers and integrated with the systems those providers use daily.

Drug Target Review
APRIL 7, 2025
As our understanding of the underlying biology of disease grows more sophisticated, emerging therapies operate on increasingly complex biopathological systems and mechanisms. Safety biomarkers account for adverse effects of a therapy under study. There are several types of biomarkers to consider.

PPD
JANUARY 15, 2025
As a result, drug developers make better decisions more quickly (removing 50% of study timeline whitespace) to bring new therapies to market faster. Accelerate customer speed to market With a modern and integrated user experience, AI solutions put the right data and insights into the right hands in real time.
Drug Target Review
JUNE 19, 2024
The mission of Lineage Cell Therapeutics is to deliver on some of the early promises of cell therapy. Cell therapy as a concept is a wonderful idea, but many of the early efforts never generated the kind of clinical data that gets people excited and leads to new medicines. Hearing aids also have all sorts of deficits.

Drug Channels
APRIL 8, 2022
To learn more about CoAssist and AssistRx specialty therapy initiation and patient support services, click here to schedule a meeting with AssistRx at the Asembia Specialty Pharmacy Summit on May 2-5, 2022. He goes on to describe four new categories of patient solutions and pharmacy models specific to this growing market segment.
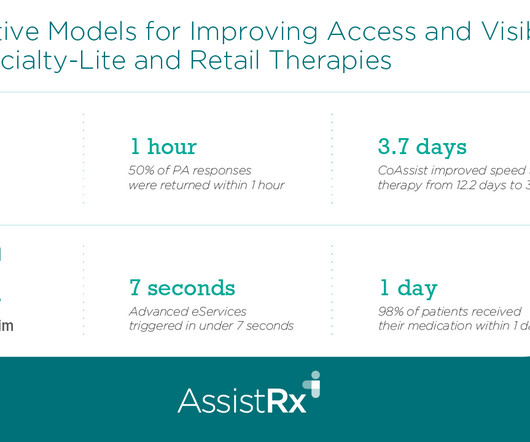
Drug Channels
OCTOBER 4, 2024
Timothy discusses the affordability and patient journey challenges of specialty-lite products for patients, manufacturers, and health care providers. He explains how AssistRx's Advanced Access Anywhere (AAA) solution streamlines processes for specialty-lite products and facilitates enrollment via a digital hub.

ASPET
OCTOBER 12, 2023
The CET RAIDR program minimizes development and procurement costs by supplementing the military medical providers' toolbox with post-Phase II therapies that demonstrate established safety and manufacturing processes, leading to a cost-sparing model for niche medicines (i.e., CBRN MCMs).

Alta Sciences
JANUARY 17, 2025
WebinarPreclinical Studies of Gene Therapy Products: Latest Trends lperez Fri, 01/17/2025 - 22:48 Image Website_webinar_Preclinical Studies Gene Therapy_v2.jpg jpg Tags Preclinical Research URL [link] Event End Date Thu, 03/06/2025 - 12:00 Event Start Date Thu, 03/06/2025 - 12:00 Weight 1
DrugBank
JANUARY 29, 2025
Gene Therapy Gene therapy operates on the principle of modulating the DNA blueprint of cells to induce a therapeutic response. In sickle cell disease, for instance, a missense mutation in the HBB gene , encoding the beta-globin subunit of hemoglobin, leads to the production of abnormal hemoglobin S.

Alta Sciences
NOVEMBER 21, 2024
Intra-Cerebroventricular (ICV) Route in Mice for Administration of Gene Therapy Products lperez Thu, 11/21/2024 - 14:35 Publication Poster_Altasciences_ACT_2024—ICV_Route_in_Mice_for_Administration_of_Gene_Therapy_Products.pdf Tags Preclinical Research Category Poster Weight 1

FDA Law Blog: Drug Discovery
NOVEMBER 2, 2023
Lewis, Senior Regulatory Device & Biologics Expert — On October 20, 2023, FDA announced the availability of the final guidance authored by CBER titled “Voluntary Consensus Standards Recognition Program for Regenerative Medicine Therapies.” A considerable roadblock in the development of RMT products is a lack of regulatory predictability.

Drug Target Review
AUGUST 6, 2024
Liver Fibrosis : Targeted therapies using ADCs could help reduce liver fibrosis and prevent the progression to cirrhosis. Expedited programs like the FDA’s Breakthrough Therapy Designation and Fast Track can shorten development timelines and reduce risk.
Drug Target Review
JUNE 9, 2023
This exclusive interview with Dr Sharon Benzeno, Chief Commercial Officer, Immune Medicine at Adaptive Biotechnologies, unveils some ground-breaking research on T- cell therapy for cancer , which has seen the first TCR-based therapeutic candidate progress to clinical development, offering promising advancements in innovative cancer treatments.
Drug Target Review
DECEMBER 5, 2024
Viral vectors have been crucial in transforming the gene therapy landscape due to their natural ability to infect cells. 1 In 2017, the US Food and Drug Administration (FDA) approved the first AAV-based gene replacement therapy (Luxturna), for Leber congenital amaurosis type 2.

Alta Sciences
NOVEMBER 4, 2024
Nonclinical Experts Discuss the Promise of Gene Therapy blussier Mon, 11/04/2024 - 21:08 HTML Driving Progress in Gene Therapy Altasciences is a leader in nonclinical research, including gene therapy. WATCH NOW Want to learn more? Speak with one of our experts.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content