Cell, gene therapy makers lose a champion at FDA with exit of Peter Marks
BioPharma Drive: Drug Pricing
MARCH 31, 2025
Marks’ resignation leaves the field without a regulator many view as “integral” to its progress over the last decade.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
MARCH 31, 2025
Marks’ resignation leaves the field without a regulator many view as “integral” to its progress over the last decade.

BioPharma Drive: Drug Pricing
DECEMBER 1, 2023
The EMA's safety committee has more questions for makers of the in-demand therapies as it reviews whether the drugs are linked to the risk of suicidal thoughts.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
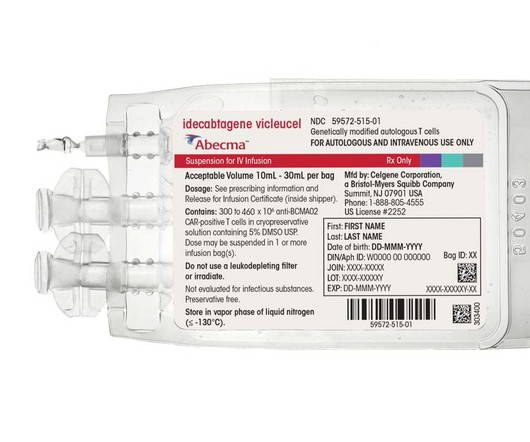
BioPharma Drive: Drug Pricing
NOVEMBER 20, 2023
The regulator plans to convene an advisory panel to discuss an expanded indication for Abecma, presenting another hurdle for Bristol Myers and partner 2seventy bio.

BioPharma Drive: Drug Pricing
JUNE 21, 2023
The regulator will issue a decision on lovo-cel by Dec. 20, roughly two weeks after a verdict is expected on a rival gene editing treatment from Vertex and CRISPR Therapeutics.

Science Daily: Pharmacology News
DECEMBER 12, 2024
Researchers have discovered a protein variant that serves as a knob for regulating the body's innate immune response. The findings could lead to new therapies for Long COVID, autoimmune disorders, and more.

Science Daily: Pharmacology News
SEPTEMBER 12, 2024
Researchers have shown unprecedented control of SIV replication and decay of viral reservoirs by combining a stringent model of infection with the interruption of antiretroviral therapy (ART).
Conversations in Drug Development Trends
NOVEMBER 12, 2024
By Amy Raymond, PhD, PMP, Executive Director, Therapeutic Strategy Lead, Rare Disease Cell and gene therapies (CGTs) include cutting-edge approaches that offer the hope of a healthier, happier, and better tomorrow for a wide range of patient populations. Below, we discuss some of these challenges in cell therapy trials.

BioPharma Drive: Drug Pricing
FEBRUARY 23, 2024
European drug regulators recommended clearing J&J and Legend Biotech's Carvykti for use as early as after first relapse, potentially giving the therapy an advantage over Bristol Myers’ Abecma.

FDA Law Blog: Drug Discovery
NOVEMBER 10, 2024
We note that the only Phase 3 study funded in the FY2024 announcement is the first-ever late-stage clinical trial for the indication of microcystic lymphatic malformations , a serious, rare genetic skin disease with no FDA-approved therapies. Relative to other areas of medicine (e.g.,

BioPharma Drive: Drug Pricing
JULY 16, 2024
The Merck KGaA spinout has a tau-regulating medicine it claims could be an “ideal therapy” for Alzheimer’s. The pitch has intrigued both healthcare investors and pharma venture arms.

Drug Target Review
APRIL 7, 2025
As our understanding of the underlying biology of disease grows more sophisticated, emerging therapies operate on increasingly complex biopathological systems and mechanisms. Safety biomarkers account for adverse effects of a therapy under study. There are several types of biomarkers to consider.

PPD
OCTOBER 17, 2024
Cell and gene therapy (CGT) studies are rapidly gaining momentum in the Asia-Pacific region, fueled by growing patient demand and a thriving ecosystem of innovation. In China, the high incidence of solid tumors is driving an urgent need for advanced therapies, spurring the push for new treatment approaches.

Drug Target Review
MARCH 4, 2025
Knowing a patients subtype or phenotype can be used to guide obesity treatments of all kinds, including drug therapy, devices, bariatric surgery and even diet and lifestyle interventions, Bagnall explains. The research demonstrated that obesity is not one disease, but many; each of which should be treated with different interventions.
Drug Target Review
AUGUST 31, 2023
Stem cell therapies have already demonstrated their prowess in treating diverse cancers and ailments linked to the blood and immune system. Consequently, the accumulation of β-amyloid not only exerts stress on other brain cells but also affects endothelial cells responsible for regulating cerebral blood flow.
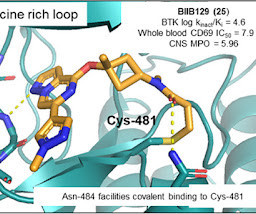
Covalent Modifiers
MAY 9, 2024
BIIB129 (25) demonstrated efficacy in disease-relevant preclinical in vivo models of B cell proliferation in the CNS, exhibits a favorable safety profile suitable for clinical development as an immunomodulating therapy for MS, and has a low projected total human daily dose.

FDA Law Blog: Drug Discovery
DECEMBER 7, 2022
Valentine — On November 22, 2022, FDA approved CSL Behring’s BLA for Hemgenix (etranacogene dezaparvovec), an AAV-based gene therapy for the treatment of adults with Hemophilia B who currently use Factor IX prophylaxis therapy, have current or historical life-threatening hemorrhage, or have repeated, serious spontaneous bleeding episodes.

ASPET
NOVEMBER 2, 2023
Spry2 is a negative regulator of receptor tyrosine kinase-related Ras/Raf/ERK pathway. The results showed that PTX-concurrent radiotherapy could aggravate fibrotic lesions in RIPF, down-regulate the content of membrane Spry2 and up-regulate the levels of p-c-Raf and p-ERK in lung tissue.

DrugBank
JUNE 13, 2024
Gene Therapy: Reprogramming the Body's Cellular Code Gene therapy is an exciting field that treats diseases at their genetic roots. A key part of gene therapy is efficiently delivering the therapeutic genetic material directly into target cells.

Chemical Biology and Drug Design
NOVEMBER 23, 2023
Multiple investigations prove that it is regulated genetically, and epigenetically, and is affected by environmental factors. The molecular and neural pathways linked to the regulation of schizophrenia have been extensively studied. Scientists are trying to use viral vectors, miRNA, siRNA, and CRISPR technology.

Drug Target Review
FEBRUARY 28, 2024
Over the past 25 years, T-cell therapies have gained significant ground in the treatment of cancer. Preclinical research on γδ T cells has made great strides since the cells were first identified in the 1980s, with γδ T-cell therapies from several companies, including IN8bio, now in or nearing clinical trials for various cancers.

Drug Target Review
DECEMBER 20, 2024
These twins simulate how a patients condition might evolve without treatment, enabling researchers to compare the real-world effects of an experimental therapy against predicted outcomes. Regulators care a lot about controlling the Type 1 error rate of the clinical trial, Smith notes.

Chemical Biology and Drug Design
JULY 4, 2023
Acacetin may be a promising drug in complementary therapy of cancer treatment. Acacetin, as a natural flavonoid drug, modulates the activity of JAK2/STAT3 pathway, to suppress the growth, migration, invasion and anti-apoptosis of cancer cells and block the progression of esophageal cancer.

Drug Target Review
SEPTEMBER 25, 2024
Most targeted cancer therapies used today operate by inhibiting targets along well-known oncogenic signalling cascades. The reactivation of oncogenic signalling upstream or downstream of the driving oncogene is a well-studied source of resistance to targeted cancer therapies.

thought leadership
JULY 1, 2024
Although this regulation applies to CTAs for gene therapy studies, it does not cover the additional requirement for a Genetically Modified Organism (GMO) application for such studies.

PPD
MARCH 11, 2025
MDT helps bridge the gap in preference heterogeneity to ensure that rare disease therapies are developed with the diverse needs of patients in mind. Challenge #4: Regulatory and market access hurdles The regulatory and health technology assessment (HTA) pathways for rare disease therapies are complex and vary by region.
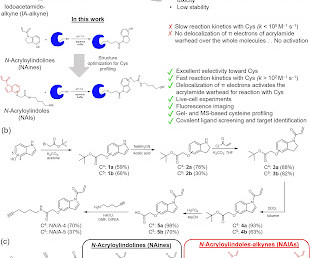
Covalent Modifiers
JULY 8, 2023
Its high sensitivity to oxidation is also important for regulating cellular processes. [link] Cysteine has been exploited as the binding site of covalent drugs.

FDA Law Blog: Biosimilars
MARCH 31, 2024
Gibbs — On March 21, 2024, the House Energy and Commerce held a subcommittee hearing titled “Evaluating Approaches to Diagnostic Test Regulation and the Impact of the FDA’s Proposed Rule.” By Ana Loloei & Jeffrey N. FDA, which was not invited to participate, would surely have concurred.

Advarra
NOVEMBER 29, 2022
The field of cell and gene therapies (CGT) is constantly evolving, and there has been significant progress in this area of research. However, despite the promise of these therapies, the regulations governing them lag the science, which in turn hinders the clinical translation of these novel medicines.

Alta Sciences
FEBRUARY 19, 2025
Beyond bioanalysis, understanding the mechanism of action is equally important, as different classes of oligonucleotidessuch as antisense oligonucleotides (ASOs)interact with mRNA in distinct ways to regulate gene expression. Our experts stay up to date on all new and evolving regulations to guarantee regulatory compliance in your studies.

Fierce BioTech
OCTOBER 2, 2023
Logistics For Cell & Gene Therapy Trials: Specific Needs Demand Special Skills Download now to learn more about intricate trial logistics, the importance of expertise, navigating regulations, secure data management, and real case studies like viral vector therapy for Spinal Muscular Atrophy.
DrugBank
JANUARY 29, 2025
Gene Therapy Gene therapy operates on the principle of modulating the DNA blueprint of cells to induce a therapeutic response. Gene therapy trials explore using lentiviral vectors to deliver a functional copy of the HBB gene to hematopoietic stem cells, the progenitors of all blood cells.

ASPET
SEPTEMBER 16, 2024
Standard of care GBM therapies include radiation and cytotoxic chemotherapy that lead to DNA damage. Various DDR inhibitors, targeting the key regulators of these pathways such as ataxia telangiectasia mutated and Rad3-related (ATR), are being explored as radio- and chemo-sensitizers.

Chemical Biology and Drug Design
AUGUST 20, 2023
Curcumin is a promising compound for the treatment of colorectal cancer or a complement of current standard therapy modalities. This study reveals the curcumin exerts antiproliferative and anti-migration effects on colorectal cancer cell lines using MACC1 as a target and inhibiting the M2 polarization of TAMs.

ASPET
MARCH 7, 2024
Increasing evidence suggests that ALDH2 is a crucial regulator of human tumor development, including HCC. Significance Statement Numerous studies have aimed to explore novel therapeutic targets for HCC, and ALDH2 has been reported to be a critical regulator of HCC progression.

Broad Institute
JUNE 10, 2024
The advance, from the lab of Broad core institute member David Liu , could one day help researchers develop a single gene therapy for diseases such as cystic fibrosis that are caused by one of hundreds or thousands of different mutations in a gene. Tags: Gene editing Gene therapy David Liu Nature Biomedical Engineering.

thought leadership
JULY 2, 2024
This EU portal is established as part of the new Clinical Trial Regulation No. The CTR is applicable to clinical trials with Gene Therapy Medicinal Products (GTMPs) as well, but unfortunately it does not cover the environmental requirements that are applicable to GTMPs if they contain or consist of genetically modified organisms (GMOs).

PPD
DECEMBER 16, 2024
Internal costs: The complexity of protocol designs a necessity for innovative therapies often requires more diverse patient populations, more extensive data collection and sophisticated trial methodologies, all of which demand higher financial outlays. Nearly 39% of sponsors cite these costs as primarily driven by complex protocols.

KIF1A
JULY 8, 2023
KIF1A-Related Research Preferential transport of synaptic vesicles across neuronal branches is regulated by the levels of the anterograde motor UNC-104/KIF1A If axons are the highways KIF1A drive on, synapses are like the off-ramps and intersections for local deliveries. What’s a pre-print? Check out this #ScienceSaturday post to learn more.

FDA Law Blog: Drug Discovery
NOVEMBER 2, 2023
Lewis, Senior Regulatory Device & Biologics Expert — On October 20, 2023, FDA announced the availability of the final guidance authored by CBER titled “Voluntary Consensus Standards Recognition Program for Regenerative Medicine Therapies.” It finalized a draft guidance published in 2022.

Drug Target Review
JULY 4, 2023
These therapies have broadened treatment options for patients to expand beyond the more traditional small molecule drug alternatives. 1 Regulators invest significant consideration balancing quality-of-life measures with overall survival when assessing novel oncology treatments. 3D rendering of Antibody Drug Conjugate Molecules.

Drug Target Review
OCTOBER 6, 2023
This master regulator of the regulatory T cell (Treg) genome has opened new horizons in the fight against cancer, offering a precise and tailored solution to modulate the immune system’s response. Can you explain how your approach compares to other therapies targeting Tregs, like checkpoint inhibitors?

PPD
MAY 7, 2024
The European Union (EU) is on the verge of a significant shift as it prepares to implement new health technology assessment (HTA) regulations in 2025. Challenges and opportunities of the new EU HTA regulation The implementation of centralized HTA presents both opportunities and challenges for pharmaceutical companies.

Advarra
NOVEMBER 14, 2024
A globally diverse DMC ensures the trial adheres to local regulations while maintaining high ethical standards. For example, consider a global Phase III, double-blind study, in a novel new therapy. Strengthened regulatory compliance: Different countries have varying regulatory requirements and expectations.

Alta Sciences
JUNE 11, 2024
My Attendance at the 2024 Boston Society Cell and Gene Therapy Conference pmjackson Tue, 06/11/2024 - 20:49 , via Wikimedia Commons" data-entity-type="file" data-entity-uuid="c7a7fa8b-b2fe-4d84-a75e-d1ba3a4e2caf" src="[link] width="742" height="249" loading="lazy" /> Ecm85, CC BY-SA 3.0
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content