Stanford University’s Innovative Medicines Accelerator and Intonation Research Laboratories form a collaboration to fight cancerous neuroendocrine tumors
SCIENMAG: Medicine & Health
JUNE 23, 2023
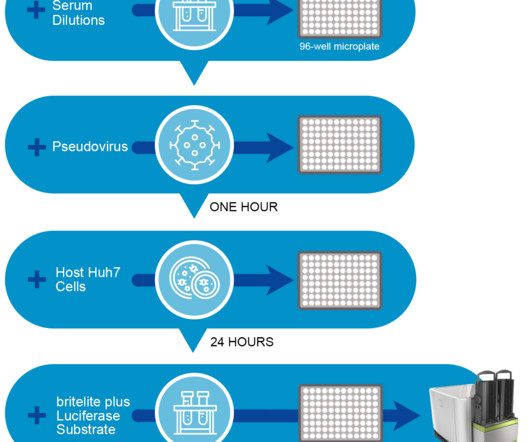
Stanford University’s Innovative Medicines Accelerator (IMA) and Intonation Research Laboratories (Intonation) have formed a collaboration to develop treatments that target cancerous neuroendocrine tumors, or tumors that form from hormone-releasing cells.











































Let's personalize your content