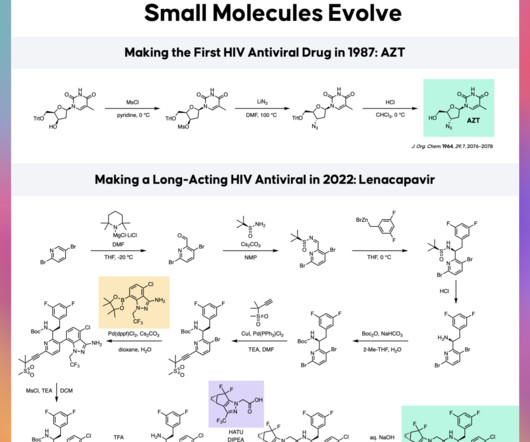
Small Molecules Evolve
Drug Hunter
JUNE 11, 2023
Small molecule drugs make up most of the drugs we take conveniently as pills, including painkillers like ibuprofen (Advil), antibiotics like penicillin and amoxicillin, or cholesterol-lowering drugs like atorvastatin (Lipitor). The small molecules drugs of today look nothing like the molecules of the 1970s.














































Let's personalize your content