New Melanoma Treatment Vaccine Shows Promise in Trial
Drugs.com
DECEMBER 15, 2023
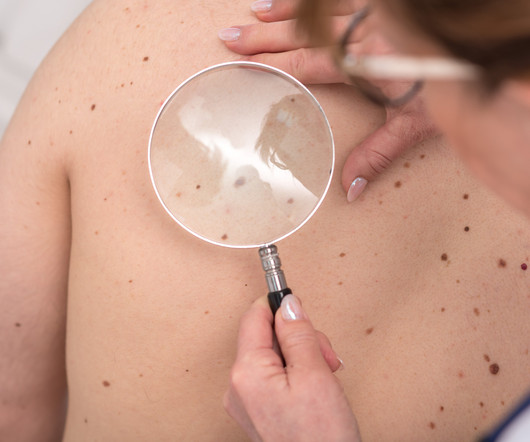
15, 2023 -- A new melanoma vaccine has shown its mettle in battling the deadly skin cancer in a new trial. People with advanced melanomas who received the vaccine plus Merck's cancer drug Keytruda were 49% less likely to die or have. FRIDAY, Dec.




































Let's personalize your content