FDA lifts pause on Novavax flu vaccine trials
BioPharma Drive: Drug Pricing
NOVEMBER 11, 2024
After an investigation, the agency concluded that a case of muscle weakness in one of Novavax’s trials was actually ALS and unrelated to vaccination.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

BioPharma Drive: Drug Pricing
NOVEMBER 11, 2024
After an investigation, the agency concluded that a case of muscle weakness in one of Novavax’s trials was actually ALS and unrelated to vaccination.

PPD
JUNE 24, 2024
In the realm of vaccine development, mega trials — studies enrolling 5,000 subjects or more — have been instrumental in the fight against many pathogens, including influenza, rotavirus, malaria, RSV and most recently in the rapid development of vaccines against COVID-19.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drugs.com
APRIL 12, 2024
FRIDAY, April 12, 2024 -- A chlamydia vaccine has triggered immune responses in an early trial, raising hopes that one day it might help curb the spread of the sexually transmitted infection (STI).There There is currently no vaccine for chlamydia, which.
Drugs.com
DECEMBER 15, 2023
15, 2023 -- A new melanoma vaccine has shown its mettle in battling the deadly skin cancer in a new trial. People with advanced melanomas who received the vaccine plus Merck's cancer drug Keytruda were 49% less likely to die or have. FRIDAY, Dec.

Science Daily: Pharmacology News
MAY 17, 2024
An HIV vaccine candidate triggered low levels of an elusive type of broadly neutralizing HIV antibodies among a small group of people enrolled in a 2019 clinical trial.

Science Daily: Pharmacology News
APRIL 29, 2024
The first study of the use of microarray patches to vaccinate children has shown that the method is safe and induces strong immune responses.
Drugs.com
JUNE 10, 2024
MONDAY, June 10, 2024 -- An experimental vaccine that could offer one-stop prevention for both COVID-19 and influenza is showing positive results among older adults in trials, maker Moderna announced Monday.The shot — for now called mRNA-1083 — "ha.

BioPharma Drive: Drug Pricing
OCTOBER 16, 2024
The hold, which was made in response to a serious adverse event report, could impact the company’s plans to start a Phase 3 trial of a combination shot for COVID-19 and influenza.
Drugs.com
AUGUST 1, 2024
1, 2024 -- A next-generation nasal vaccine for COVID-19 appears to do what injectable vaccines can’t -- actually stop the spread of the virus from person to person.Hamsters that received the nasal vaccine didn’t pass the virus on to. THURSDAY, Aug.

Science Daily: Pharmacology News
OCTOBER 17, 2024
A new vaccine provides hope for treating and even preventing the highly contagious and difficult-to-treat Clostridioides difficile infection, more commonly known as C. difficile vaccine was found to protect against C. difficile vaccine was found to protect against C. difficile or C. In animal models, this first mRNA-LNP C.
Drugs.com
SEPTEMBER 4, 2024
4, 2024 -- Current vaccines against mpox were designed to fight an older, rarer cousin of the virus, smallpox.Now, new research from the drug company Moderna suggests its new mpox vaccine, based on mRNA technology, might do a. WEDNESDAY, Sept.

Drugs.com
OCTOBER 6, 2023
announced Wednesday that it has seen positive early results with a new vaccine that would guard against four strains of flu plus COVID-19. In interim findings from a Phase 1/2 trial, the vaccine showed both a. THURSDAY, Oct. 5, 2023 -- Moderna Inc.
Drugs.com
OCTOBER 11, 2023
11, 2023 -- New research points to the potential of a COVID-19 vaccine delivered through the nose. The phase 1 clinical trial showed that the product, administered nasally in two doses, delivered a significant immune response to. WEDNESDAY, Oct.

Drug Discovery Today
DECEMBER 22, 2020
First in human trials for vaccine candidate with the potential to confer long-term immunity

Drugs.com
MAY 2, 2024
THURSDAY, May 2, 2024 -- An experimental cancer vaccine can quickly reprogram a person’s immune system to attack glioblastoma, the most aggressive and lethal form of brain cancer, a small, preliminary study has found.The cancer vaccine is based.
Drugs.com
APRIL 8, 2024
MONDAY, April 8, 2024 -- A pancreatic cancer vaccine has continued to protect a small group of patients from their cancer coming back, three years after receiving the jab, a new study says.Eight patients have not had their pancreatic cancer recur.
SCIENMAG: Medicine & Health
SEPTEMBER 15, 2023
Enrollment in a Phase 1 trial of a new investigational universal influenza vaccine candidate has begun at the National Institutes of Health’s Clinical Center in Bethesda, Maryland.

Drug Discovery Today
APRIL 15, 2021
First subjects dosed with AKS-452, COVID-19 vaccine candidate in the Netherlands trial the vaccine is shelf-stable for 4 months at 25 degrees Celsius (77° Fahrenheit). 176 volunteers will participate in the clinical trial the safety and immune response read-outs expected in Q2 2021
Drugs.com
APRIL 9, 2024
TUESDAY, April 9, 2024 -- A custom-made anti-tumor vaccine added to standard immunotherapy was twice as likely to shrink liver cancer as when a patient received immunotherapy alone, a new study shows.The vaccine could help liver cancer patients.

SCIENMAG: Medicine & Health
AUGUST 3, 2023
The Access to Advanced Health Institute Receives $18 Million Award to Develop a Temperature Stable, Single-Dose Chikungunya RNA Vaccine Through a Phase 1 Clinical Trial Credit: Delaney Brown Photography The Access to Advanced Health Institute Receives $18 Million Award to Develop a Temperature Stable, Single-Dose Chikungunya RNA Vaccine Through a Phase (..)

DrugBank
JULY 18, 2024
Vaccines have consistently demonstrated their efficacy in protecting people from infectious diseases. Yet, the recent COVID-19 pandemic highlighted the obstacles that are inherent today in the development of vaccines. Stabilizers play a crucial role in maintaining the vaccine's potency and integrity throughout its shelf life.

BioPharma Drive: Drug Pricing
DECEMBER 5, 2023
The announcement ends a lengthy setback that began when the company and partner Valneva accused a clinical trial site operator of study misconduct.
Drugs.com
JANUARY 9, 2024
9, 2024 -- Patients with the most common form of pancreatic cancer could benefit from an experimental therapeutic vaccine, a small new clinical trial shows.The vaccine, called ELI-002 for now, is targeted to what are known as. TUESDAY, Jan.9,

Fierce BioTech
JUNE 12, 2024
By Ayako Wakatsuki Pedersen, SVP of Translational Research at IO Biotech | Historically, most cancer vaccine trials have fallen short. What is the potential of new vaccines that can dismantle cancer cells' defenses?

SCIENMAG: Medicine & Health
JUNE 12, 2023
Peer-reviewed / Randomised Controlled Trial / People Study of healthy US adults found that a single dose of the VLA1553 vaccine candidate was generally safe, well tolerated and provokes an immune response.
The Pharma Data
SEPTEMBER 25, 2020
A trial of a new vaccine that appears to train the immune system to fight coronavirus has begun in the UK. The trial on 10,000 people will now see if the vaccine can prevent people getting ill. Some of the vaccines being developed for Covid-19 use either completely new or barely proven technologies. .

BioPharma Drive: Drug Pricing
JULY 8, 2024
Shares in HilleVax plummeted after the company reported its experimental shot failed to meet the primary and secondary goals of the Phase 2b study.

The Pharma Data
OCTOBER 22, 2020
COVID-19 Vaccine Trial Enrolls 30,000. 22, 2020 — The first company to start a phase 3 trial of a COVID-19 vaccine reached its target of enrolling 30,000 participants, CNN reported Thursday. Half of the participants were given the vaccine and half received a placebo. trial in August. Professional.
The Pharma Data
SEPTEMBER 14, 2020
. AstraZeneca has confirmed that clinical trials assessing its Oxford University partnered coronavirus vaccine AZD1222 have resumed in the UK, following a green light by the Medicines Health Regulatory Authority (MHRA). Source link.

PPD
JULY 6, 2023
It’s estimated that nearly three out of every four clinical trials are conducted by contract research organizations (CROs), highlighting just how much sponsors value — and rely on — the work that CROs perform. Surveys show that CROs improve trial efficiency and increase productivity.
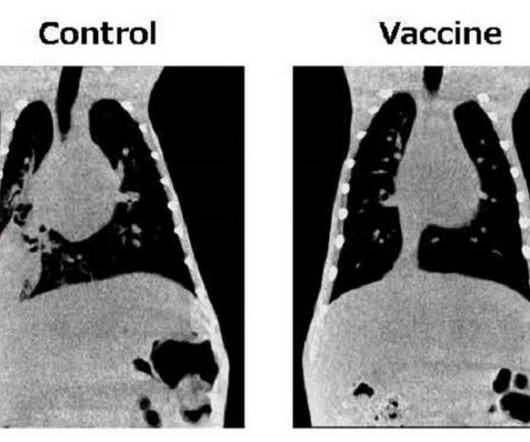
SCIENMAG: Medicine & Health
NOVEMBER 27, 2023
Osaka, Japan – The global impact of the coronavirus pandemic has ignited a renewed focus on emerging and re-emerging infectious diseases. Researchers at Osaka Metropolitan University are making great strides in combating pneumococcal pneumonia, one of the leading causes of respiratory deaths worldwide.

Advarra
JANUARY 11, 2024
While mRNA usage has played several roles in clinical research , oncology researchers in particular are eager to explore the possibilities of mRNA-based cancer vaccines. The mRNA constructs used in COVID-19 vaccines, for example, direct cells to produce a version of the “spike” protein studding the surface of SARS-CoV-2.

Alta Sciences
SEPTEMBER 27, 2024
Featuring two scenarios that explore the complexities of bioanalysis for immunomodulators, The Altascientist offers practical considerations for ensuring accurate bioanalysis, as well as pharmacokinetic, pharmacodynamic , and safety data in clinical trials. Each class of immunomodulator has a defined complexity and mechanism of action.
The Pharma Data
OCTOBER 26, 2020
COVID-19 Vaccine Trials. 26, 2020 — Drugmakers AstraZeneca and Johnson & Johnson are ready to resume paused COVID-19 vaccine trials after health scares, CNN reported. The AstraZeneca trial was stopped in early September, and the Johnson & Johnson trial was paused earlier this month.

The Pharma Data
JUNE 26, 2021
Sanofi and Translate Bio initiate Phase 1 clinical trial of mRNA influenza vaccine. The trial will evaluate the safety and immunogenicity of a monovalent flu vaccine candidate coding for the hemagglutinin protein of the A/H3N2 strain of the influenza virus. JUNE 22 , 2021.
The Pharma Data
DECEMBER 8, 2020
8, 2020 — AstraZeneca’s COVID-19 vaccine is safe and effective, new data from late-stage trials shows. Overall, the vaccine protected against symptomatic disease in 70% of cases, according to a team led by researchers from Oxford University in England. TUESDAY, Dec. 8 in The Lancet.
The Pharma Data
SEPTEMBER 2, 2020
. Sanofi and GSK have begun a Phase I/II clinical trial testing their adjuvanted COVID-19 vaccine in healthy adults. The vaccine candidate, developed in partnership by the firms, is based on the recombinant protein-based technology used in Sanofi’s seasonal influenza vaccines and GSK’s pandemic adjuvant technology.

The Pharma Data
NOVEMBER 19, 2020
Russia’s Gamaleya Research Institute has reportedly resumed dosing in a phase 3 trial evaluating its COVID-19 vaccine, Sputnik V., but a Russian government spokesperson denies that the study was ever paused. Source link.
The Pharma Data
SEPTEMBER 7, 2021
The first patients have been enrolled in a phase 1 randomized placebo-controlled clinical trial to study a therapeutic vaccine for opioid use disorder developed by researchers at the University of Minnesota Medical School. The vaccine currently being tested stimulates the body’s immune system to produce antibodies to oxycodone.

The Pharma Data
NOVEMBER 2, 2020
German biopharma company CureVac announced yesterday that its COVID-19 vaccine hopeful generated immune responses and was generally well-tolerated in phase 1 trial participants, lending support for a pivotal trial before year’s end. Source link.
The Pharma Data
NOVEMBER 18, 2020
18, 2020 — Children should be included in clinical trials for a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine at the earliest stages, according to a letter from the president of the American Academy of Pediatrics (AAP), on behalf of more than 67,000 pediatricians and pediatric medical and surgical subspecialists.

The Pharma Data
JULY 21, 2021
Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company focused on the development and commercialization of prophylactic vaccines for infectious diseases with significant unmet medical need, and Pfizer Inc. If successful, this trial could enable the inclusion of a pediatric population in the Phase 3 trial.

PPD
MAY 11, 2023
The global COVID-19 pandemic increased awareness of the importance of vaccine development — both for drug developers and the public. The speed at which COVID-19 vaccines were developed was remarkable, but like most newly developed vaccines, there was variation among who could receive the shots and when.

The Pharma Data
SEPTEMBER 9, 2020
Global trials for AstraZeneca’s much-hyped COVID-19 vaccine, currently in development in partnership with the University of Oxford, have been halted after an unexplained illness was identified in one of the participants. In large trials illnesses will happen by chance but must be independently reviewed to check this carefully.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content